Persistence of Change
Environmental exposures can alter gene activity for anywhere from minutes to a lifetime. Some changes might even persist over generations.
For the past five years, every time Wan-Yee (Winnie) Tang has traveled to Beijing or Shanghai, she has returned home with a hacking cough.
These cities’ legendary smog—a combination of particulate pollution from millions of cars as well as factories and coal-fired power plants, contains a mix of chemicals that are dangerous to breathe.
“No matter what I do, I just can’t stop coughing,” she says.
After a few days in the relative clean of Baltimore’s air, her cough clears up—until the next time she crosses the Pacific. For Tang, PhD, BSc, who studies the effects of air pollutants on asthma development in Environmental Health and Engineering (EHE) at the Bloomberg School, this up-close and personal display of how toxic particles can lead to breathing issues provides a dramatic example of how quickly an environmental exposure can cause problems. If she can develop a cough over just a few days, what must this contaminated air be doing to those who breathe it every day?
It’s these long-term effects that Tang is trying to study: What happens to children years or even decades after being exposed to air pollutants—such as diesel fumes, particulate matter or cigarette smoke—in the womb? Epidemiological studies have linked diesel pollution and maternal smoking to childhood asthma and other pulmonary illnesses. Tang has spent the last decade trying to understand these connections.
Her field is known as epigenetics, the study of processes that affect gene regulation without changing the underlying DNA code. Many environmental exposures create health problems via epigenetic changes that alter which genes are switched on and off. These modifications can last for anywhere from a few minutes to decades.
Now, new research from Tang and other scientists reveals that some of these changes may persist over multiple generations, meaning a mother’s smoking could affect not only her child’s health, but also her grandchild’s. The evidence has been shown only in preliminary animal studies, although some human studies hint that the phenomenon is occurring in us, too. The idea continues to be hotly contested at conferences and in research journals. If confirmed, however, it could change how scientists think about the dangers of environmental exposures.
“If we can intervene and get a mother to stop smoking, we won’t just be helping one generation, we’ll be helping many,” Tang says.
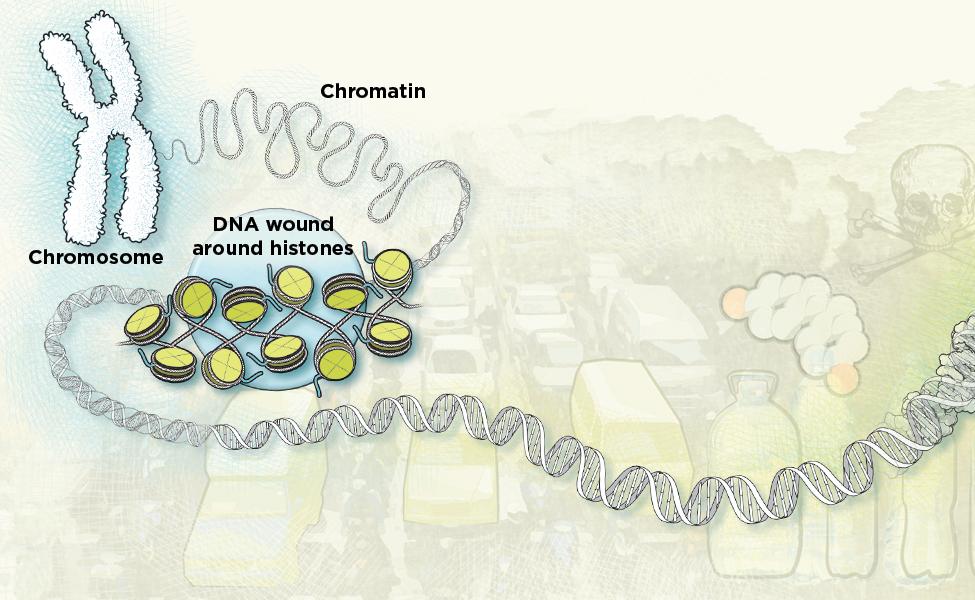
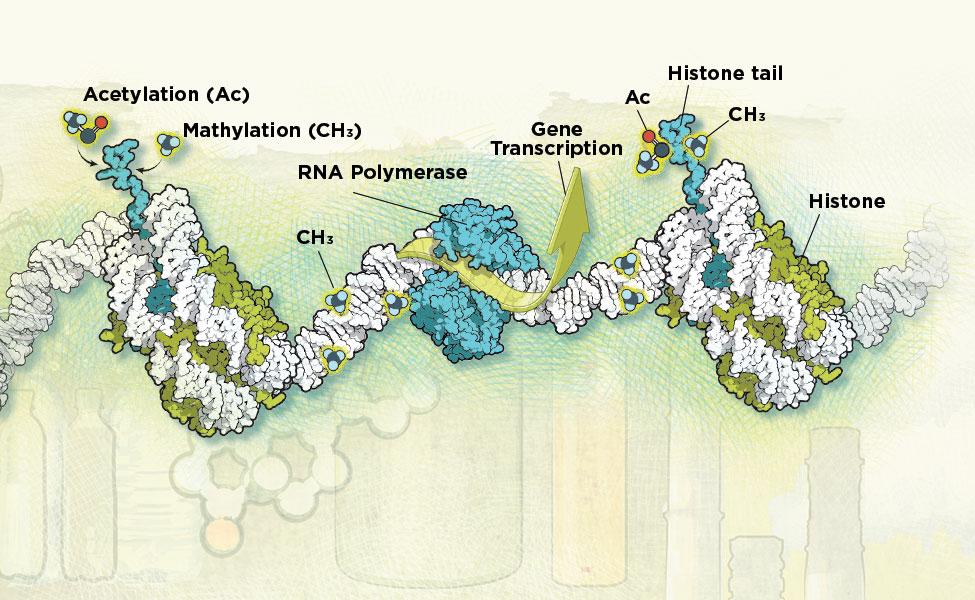
In the 1940s and ’50s, British scientist Conrad Waddington coined the term “epigenetics” to explain how the cells in an early embryo, all of which share the same DNA, can differentiate into forms as wildly different as the skin cells on your feet and the neurons in your brain. The organism’s genetic code didn’t change; instead, what changed was how those genes were regulated, with some turned on and others switched off. Later work began elucidating how this happened. Enzymes can attach small chemical tags called methyl groups at specific DNA sequences in the genome. These tags act as a volume control for a gene—either amplifying its effects by allowing more of the cell’s transcription and translation machinery to turn it into protein, or dialing down transcription and translation. Methyl and acetyl groups can also tag histones, small protein beads around which the double helix of DNA wraps itself. Some of these chemical tags alter gene activity only for a few minutes or hours; others may last for years—even a lifetime.
Although Waddington focused on developmental biology, the field of epigenetics soon expanded into other areas of medicine, including oncology and environmental health.
“When people breathe in harmful air and get a disease, this isn’t changing their DNA. What’s changing is their epigenome,” says Zhibin Wang, PhD, an EHE assistant professor.
For decades, however, researchers believed that shortly after fertilization, epigenetic tags were cleared from a zygote’s genome, meaning that epigenetic changes would come and go within a single generation. Animal studies from several different institutions have more recently indicated that not all epigenetic markers may be erased. Scientists documented epigenetic changes that persisted for four or more generations in rats and other laboratory animals.
“This is really hard to do in humans. It’s hard to track these exposures, and it takes a really long time,” says Xiaobin Wang, MD, ScD ’91, MPH, director of the Center on the Early Life Origins of Disease at the Bloomberg School. Wang and her team have studied epigenetics in the context of prenatal environmental exposures and child health outcomes in the Boston Birth Cohort for over a decade, and have collaborated with Tang on several projects.
Several years ago, endocrine-disrupting chemicals like bisphenol A (BPA) were in the news for their ability to wreak havoc on human and animal reproductive systems. Tang’s work has begun to examine the links between BPA and later-life prostate cancer in rats. Male rats exposed to BPA neonatally showed changes in the DNA methylation of more than 100 genes, including seven genes linked to decreased survival in human prostate cancer patients. In humans, Tang and Xiaobin Wang found that prenatal exposure via ingestion and inhalation to a group of commonly used flame retardants decreased the methylation of the gene for tumor necrosis factor alpha, a protein associated with immune-related disorders and cancer. In another study, Tang found that maternal inhalation of chemicals called polycyclic aromatic hydrocarbons, which form as a byproduct of combustion and incineration, altered cord blood DNA methylation patterns in offspring.
Showing that these epigenetic markers can be transmitted through multiple generations in humans will be no easy task, as Tang well knows. It’s why she has used animal models to investigate how these changes might happen and whether they persist. To identify epigenetic markers in humans that may be acquired during development, researchers like Xiaobin Wang and Christine Ladd-Acosta, PhD, a genetic epidemiologist at the Wendy Klag Center for Autism and Developmental Disabilities, have begun documenting how prenatal environmental exposures can shape epigenetics over the lifespan. Both researchers have conducted studies to show how various environmental exposures can alter a fetus’s epigenetics—effects that can be detected into childhood.
The most challenging part of these studies has not been measuring the epigenetic marks but nailing down the prenatal exposures. This is why Ladd-Acosta and her colleague M. Daniele Fallin, PhD, chair of Mental Health and director of the Wendy Klag Center, first examined prenatal maternal smoking for work on exposure-related epigenetic changes. Using data they had gathered in one of their autism studies, they examined a subset of 572 children ages 3 to 5, and although they didn’t suspect a connection between smoking and autism, they wanted to explore the effects of smoking.
“People don’t always know what was in the air they’re breathing, but they do remember if they smoked,” Ladd-Acosta says.
Every few years, they drew blood from the children to measure the methylation of their DNA. Although some of the epigenetic marks of prenatal smoke exposure disappeared over time, not all of them did. This work, published in Environmental Research in 2016, identified an epigenetic signature of prenatal exposure to cigarette smoke, and opened the door for the use of epigenetics to determine whether an individual has been exposed to any number of environmental contaminants.
Xiaobin Wang has relied on data from the Boston Birth Cohort. The BBC has tracked more than 8,500 mother-child pairs from predominantly low-income, minority populations for nearly two decades. Wang, who built the study from the ground up, had the foresight to save DNA samples from both the mothers and their babies’ umbilical cords, which preserved the epigenetic markers present at birth. Their work trying to identify causes of preterm birth found both epigenetic and genetic differences in preemies compared to babies carried to term.
“Our data suggest that genetics, epigenetics and environment are working together,” Wang says. Wang and other scientists continue to explore how these factors interact to contribute to preterm birth and other child health problems.
This work, the researchers stressed, is preliminary. No one yet has enough data to say how common transgenerational epigenetic inheritance is. Some, like Kasper Hansen, PhD, an assistant professor in Biostatistics, remain dubious that the phenomenon is even real. “Researchers still have to eliminate a lot of other possibilities before they can say it’s really happening,” Hansen says.
Tang admits that conclusive proof can come only from long-term human studies. Still, increasing numbers of animal studies continue to provide evidence, and Bloomberg School scientists are working to replicate those studies in humans. As the BBC approaches its 20th birthday, Xiaobin Wang has begun following the grandchildren of the original women in the study, which includes gathering epigenetic data. And Tang continues work on her animal models to explain why certain environmental exposures alter methylation of certain genes.
“I think we’ll eventually see evidence for this in humans, but it might take a while,” says Zhibin Wang.
Their work has the potential to provide a new understanding of how today’s environmental pollution can haunt humanity for generations to come. No longer can we treat environmental issues as temporary problems that can, with enough resources, be remediated and forgotten. Instead, these exposures could manifest in the human genome far into the future, which makes it even more crucial for scientists to understand—and prevent—these harms.