Mini-Brains Help Uncover COVID-19’s Neurological Impact
Lab-grown organoids show how SARS-CoV-2 infects brain cells.
In March, reports began to emerge that COVID-19 may affect the brain as well as the lungs and heart, adding to the miasma of fear and uncertainty surrounding the new disease.
Fortunately, Thomas Hartung has developed a powerful experimental model for clarifying SARS-CoV-2’s neurological impact.
It is exceedingly difficult to accurately model the human brain in the laboratory. Rats and mice offer useful starting points, but there is no replacement for human tissue. “Unfortunately, nobody is willing to make a donation,” jokes Hartung, MD, PhD, a professor in Environmental Health and Engineering and director of the Center for Alternatives to Animal Testing. Thus, most specimens come from patients who have died or undergone surgery for an existing disease.
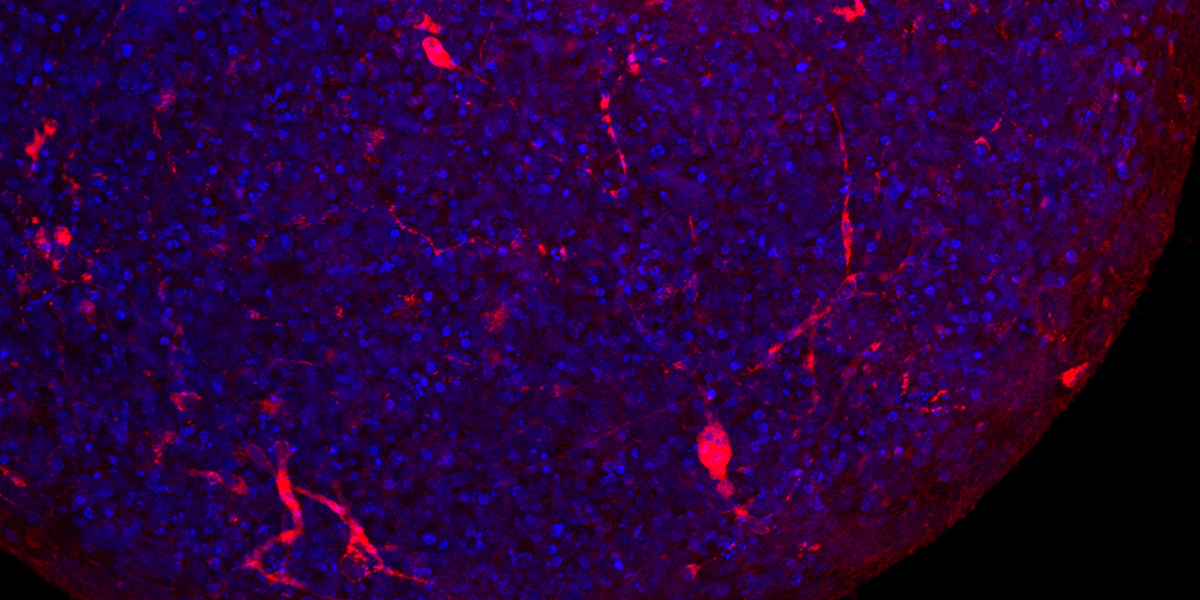
In 2016, Hartung’s team devised a solution based on what are known as induced pluripotent stem cells. Derived from genetically reprogrammed skin cells from adult donors, these cells can, like embryonic stem cells, produce virtually any other cell type. Hartung’s team created a strategy for coaxing these cells to form brain “organoids”—tiny balls of neurons and other cells that replicate key features of the human brain.
Having used “mini-brains” to study viruses like Zika, his team recognized a clear opportunity to apply their technology to COVID-19. In a June study in ALTEX: Alternatives to Animal Experimentation, Hartung and colleagues offered the first substantive evidence that SARS-CoV-2 can infect the brain. “Some brain cells were clearly infected [and] the virus had multiplied a hundred- or thousand-fold in these cells,” he says, adding they also saw evidence of the virus spreading within the organoid.
Organoid models are limited in that they more closely resemble the fetal brain than an adult’s. But Hartung sees this as an asset and is exploring whether the virus poses a threat to pregnant women and their unborn children. “Our current research is focused on whether neural development is derailed,” he says. If so, that could lead to lifelong conditions like autism or attention deficit hyperactivity disorder.
Thomas Zwaka, MD, PhD, a cell biologist at Icahn School of Medicine at Mount Sinai who has worked extensively with organoids, sees this modeling effort as an important step in understanding the still-novel disease. “This kind of work informs clinicians,” he says. “Everybody is currently focused on the lung and digestive system, but … the ability to look in brain organoids at what cells are infected and what the virus does to these neurons is important.”