Disarming a Brain Bomb: 3D Studies of Toxoplasma May Help Neutralize a Common Parasite
A small-intestine organoid allows Isabelle Coppens to study Toxoplasma in three dimensions—and test ways to kill it before it can harm its host.
Isabelle Coppens waited in the bustling café on the second floor of the Wolfe Street building, anxiously studying each new face. Then, at precisely 3 p.m. on September 25, 2017, Gabsang Lee arrived. Despite working less than a block from each other, neither scientist had heard of the other until an internet search by Lee identified Coppens as a potential collaborator.
Sipping her Diet Coke, Coppens, PhD, MSc, a professor in Molecular Microbiology and Immunology, listened as Lee proposed his plan. Lee’s work on stem cells in the Department of Neurology and Neuroscience at the Johns Hopkins School of Medicine had led him to build an enteroid—a three-dimensional miniature small intestine—as part of his work to study how cells mature during development.
About the size of a grain of sand, Lee’s mini-guts more closely mimicked the biology of the human gut than standard cell cultures. Months of painstaking work in a tiny corner lab had created a sphere of cells, but Lee had no way of knowing if the cluster of cells he saw under the microscope were identical to those found in the small intestine. One way to verify this? Infect the cells with a parasite that could only infect the small intestine.

Through his training as a veterinarian, Lee was familiar with a common parasite called Toxoplasma gondii that infects intestinal cells called enterocytes in both cats and humans. The parasite is easy to grow in the lab, and infection happens quickly—the perfect choice to confirm whether Lee had, in fact, built an enteroid. A quick search for nearby Toxoplasma experts revealed a single name: Isabelle Coppens.
Coppens had spent most of her career studying T. gondii, the parasite that causes toxoplasmosis. Though Toxoplasma is most notorious as the reason pregnant women should avoid cat litter (in case Fluffy’s feces are infected), toxoplasmosis can cause deadly encephalitis in people with weak immune systems. Toxoplasma can infect most mammals, and its life cycle begins in the small intestine—the exact organ Lee was building in his lab. If the T. gondii Coppens grew in her lab could infect the enteroid, Lee would know his experiments had worked.
“I can’t wait to infect your organoids,” Coppens said.
As the two scientists discussed their options, Coppens and Lee realized that they had an opportunity to go far beyond a simple test of an enteroid. The flat, two-dimensional cell cultures Coppens had used to study Toxoplasma over the previous 20 years produced lots of parasites—but the infected cultured cells looked nothing like infected cells in humans.
“It’s flat biology, and flat biology doesn’t exist in our body,” Lee says.
Coppens was more than happy to share her stock of T. gondii, but she proposed a broader collaboration: using Lee’s enteroids to study Toxoplasma in a system that more closely matched the biology of infected humans. Lee immediately agreed.
“It was a real lucky case for us, to have someone in the next building who works with Toxoplasma,” Lee says.
Their proposed project is ambitious. Besides improving our understanding of Toxoplasma’s basic biology, the enteroids will allow Coppens and Lee to screen potential drugs for their ability to halt Toxoplasma in its tracks. It’s too early to say whether the plan will work. But if it does, it promises to bring the first new treatment for toxoplasmosis in more than a half century.
A Parasitic Time Bomb
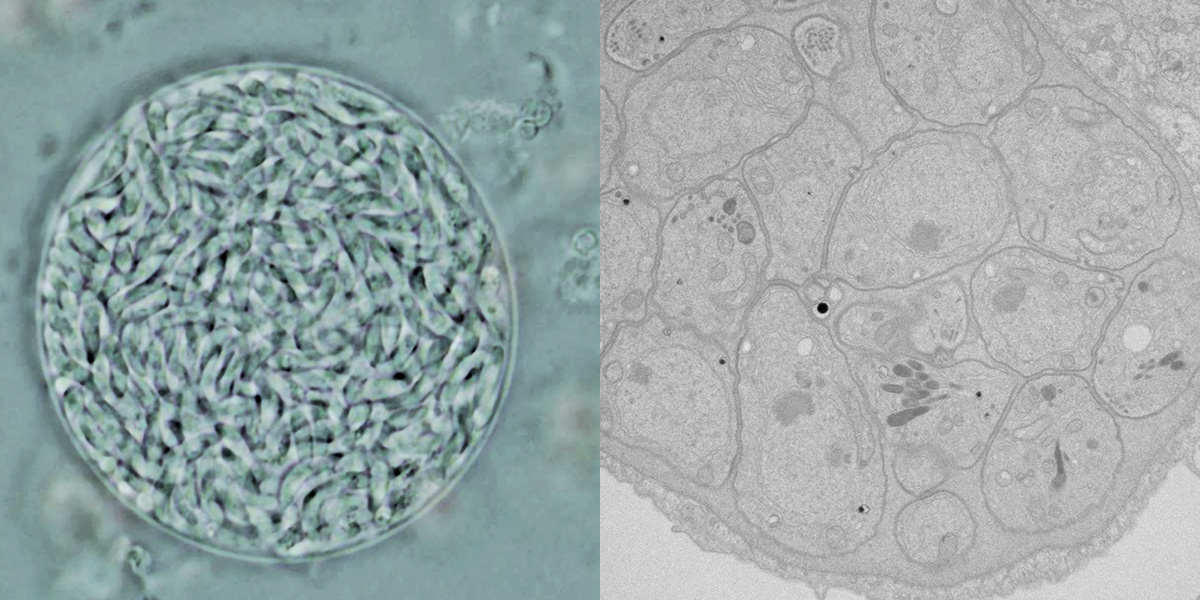
Toxoplasma’s cosmopolitan tastes mean it can survive in water, soil, pretty much any mammalian cell and, yes, cat poop. But Toxoplasma can also be persnickety, especially when it comes to sex. For this parasite, romance takes guts—specifically feline small intestines. Though the parasite can and does produce trillions of copies of itself when it infects a warm-blooded host, cat guts are the only place where Toxoplasma can reproduce sexually. Once safely ensconced in feline enterocytes, the parasites form gametes and recombine into a brand-new Toxoplasma in the form of a thick-walled cell called an oocyst. This oocyst breaks open the enterocyte where it was born and exits the cat body in feces. From here, it can infect any warm-blooded animal that swallows the oocyst, which once again enters the cells of the small intestine and transforms into a fast-dividing form known as a tachyzoite that spreads throughout the body. When tachyzoites reach brain and muscle cells, they barricade themselves into hardy cysts.
For most healthy people, initial infection with Toxoplasma may create only mild flu-like symptoms. As long as the immune system remains intact, an infected host can live in relative harmony with Toxoplasma. But if the immune system gets disrupted—by HIV, cancer chemotherapy or anti-rejection drugs for organ transplants, for example—the parasite can burst free from the cyst and replicate unchecked in the brain, leading to blindness, encephalitis and even death. Global statistics on the number of deaths due to toxoplasmosis are hard to come by, likely because many deaths occur in resource-poor settings and the symptoms of toxoplasmosis don’t differentiate it from other forms of encephalitis.
“It’s like a bomb you have in the brain,” Coppens says.
It’s a bomb she has in her own brain. Around the world, one-third of the global population (2.5 billion humans) have been infected with Toxoplasma, making this one of the most common human infections. In French-speaking countries, like the part of Belgium where Coppens grew up, infection rates top 90 percent. The parasite can thank the French love of steak tartare.
“It is just raw meat that we mix with a yolk of an egg and onion. It’s so good,” she raves.
A Portrait of the Scientist
Shaped like a swollen, slightly curved sesame seed (toxo is Greek for “arc”), the infective form of Toxoplasma is just one-tenth the width of a human hair. As an obligate intracellular parasite, Toxoplasma can replicate only within the confines of a host cell. Ready access to its host’s energy stores, however, has made Toxoplasma the consummate freeloader. Over time, Toxoplasma has lost the ability to synthesize everything from vital amino acids to energy-rich fatty acids, leaving a pared-down genome full of the tricks it needs to infect a host, make millions of copies of itself and then hunker down for years or even decades.
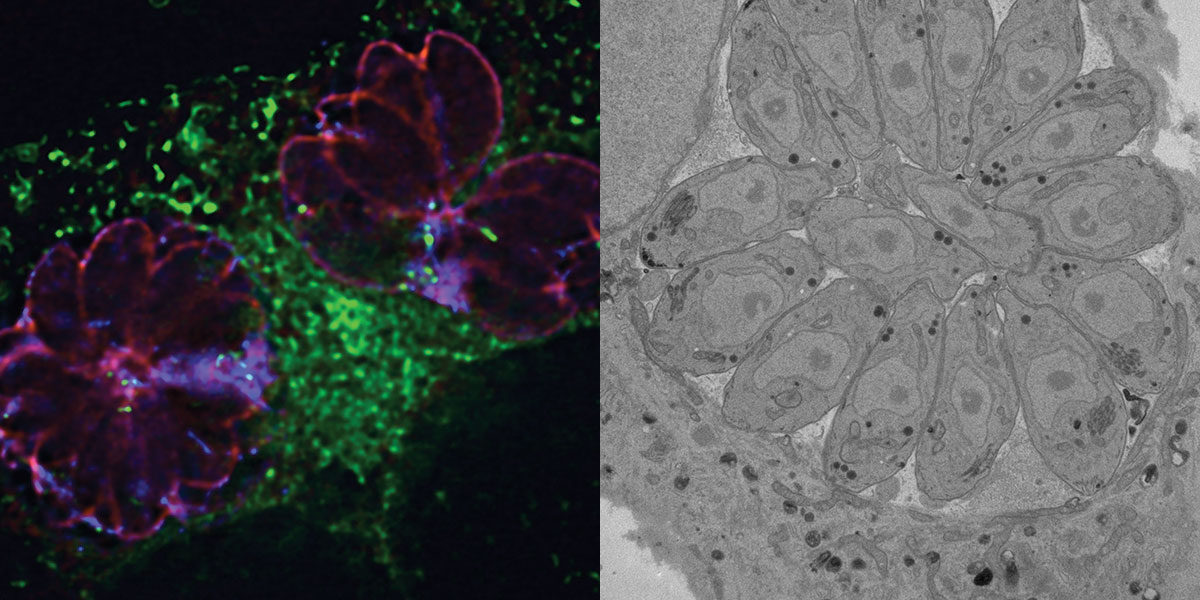
When Toxoplasma enters a host cell, it wraps itself in the host plasma membrane and creates a parasitic vacuole, a membrane-enclosed space within the cell that provides a safe place for Toxoplasma. Mammalian cells can readily recognize the presence of an intruder. But Toxoplasma can go almost completely incognito, avoiding destruction by harmful lysosomes, the stomach of the cell. To survive in the host cell, the parasite must hijack cellular organelles, rich reservoirs of food, to fuel its own breakneck growth as well as consume their contents to filch molecular building blocks like proteins and lipids.
At her desk three stories above the café where she first met with Lee, Coppens proudly shows off microscopic images of a vacuole’s thin tendrils lassoing the infected cell’s organelles including lysosomes and pulling them closer to Toxoplasma. She used a transmission electron microscope to take these images, cutting wafer-thin sections of infected cells with a diamond knife and then bombarding the sections with beams of electrons to capture details too small to see with even the most powerful light microscope. Until Coppens took those images, no one knew whether the parasitic vacuole gathered nutrients from the host by doing the equivalent of sieving the cellular soup through a cheesecloth or using a straw to suck up specific molecules. The ghostly tendrils that connected the vacuole to cellular organelles revealed a cobweb of direct linkages.
“Isabelle’s research defining how parasites steal resources from their hosts—including humans—is probing the very essence of parasitism,” says Vernon Carruthers, a microbiologist at the University of Michigan who has collaborated with Coppens on several Toxoplasma studies. “Using a potent blend of genetics and microscopy to visualize the parasite’s clever feeding strategies, her lab is creating a detailed photo album of a microscopic heist.”

A Glutton and a Thief
In Coppens’s cell cultures, a single tachyzoite can divide to fill a parasitic vacuole with upward of 50 Toxoplasma in just 36 hours. Mammalian cells can contain several vacuoles, with individual Toxoplasma arrayed like a rosette. The vacuoles swell larger and larger until the cell finally bursts. Less than two days later, the parasites have consumed the entire host cell. The only remnants are tiny pieces of debris that float in the culture dish like flakes of dandruff. “There’s nothing left,” Coppens says.
In humans, it takes three times as long for the cells to explode, but the process is the same, she says. Key to their explosive growth? Lipids like cholesterol and the fatty acid oleate.
“Toxoplasma is a glutton,” Coppens says. Her French accent makes her pronounce glutton as glue-TOHN, giving an elegant air to what is essentially the molecular equivalent of gnawing on a stick of butter. “It is very greedy,” she points out. And in this gluttony, Coppens may have uncovered Toxoplasma’s Achilles’ heel.
If you feed a human cell too much fat, it will store some of the excess in tiny lipid droplets that help to buffer it against future lean times. So, too, will Toxoplasma. Keep overfeeding most types of cells, and eventually they will simply stop accepting lipids. Not Toxoplasma. “The more you give it, the more it will take,” Coppens says.
“If you put the parasite in another environment which is extremely rich, like an adipocyte,” she pauses, then gasps in mock awe. “It’s paradise. It will eat like hell.”
Her August 2018 paper in Antimicrobial Agents and Chemotherapy shows evidence of this process in action. When Coppens and colleagues added extra unsaturated fatty acids to cell cultures infected with Toxoplasma, the parasite slurped up so many lipids it puffed up and stopped growing, showing signs of serious distress. Coppens then dosed infected cells, now swollen like grease-filled balloons, with T863, a drug initially developed to treat obesity and diabetes by blocking lipid storage. T863 made Toxoplasma even worse at handling the influx of fat. When the researchers gave both unsaturated fat and T863 to a Toxoplasma culture, it provided a one-two punch that killed the parasite by preventing the cell’s mitochondria from converting the stored fats into energy. Coppens still doesn’t know whether T863 will also help kill the slow-growing Toxoplasma in cysts in the brain, but she hopes to test that idea soon.
“Nobody else was paying attention to this process until I took a look,” Coppens says, shaking her head in disbelief.
“Dr. Coppens’s groundbreaking work on Toxoplasma is teaching us why this organism is such a successful parasite,” says Arturo Casadevell, MD, PhD, chair of Molecular Microbiology and Immunology.
Enter the Enteroid
The work with unsaturated fat and T863 provides a major step forward for the development of an effective Toxoplasma treatment, but the standard cell cultures Coppens uses in her research can’t capture how the parasite behaves in humans. Lee’s enteroids come much closer. It took Lee’s postdoc Hojae Lee more than a year to concoct the perfect chemical stew to transform stem cells into the hollow sphere that is an enteroid. Culturing small intestine cells in two dimensions is challenging; adding another dimension made the process even more difficult. As in standard cell cultures, Hojae had to feed the cells every other day, no exceptions. He also needed to add expensive chemicals like growth factors in precise amounts at exactly the right time in order to coax the stem cells into their final form.
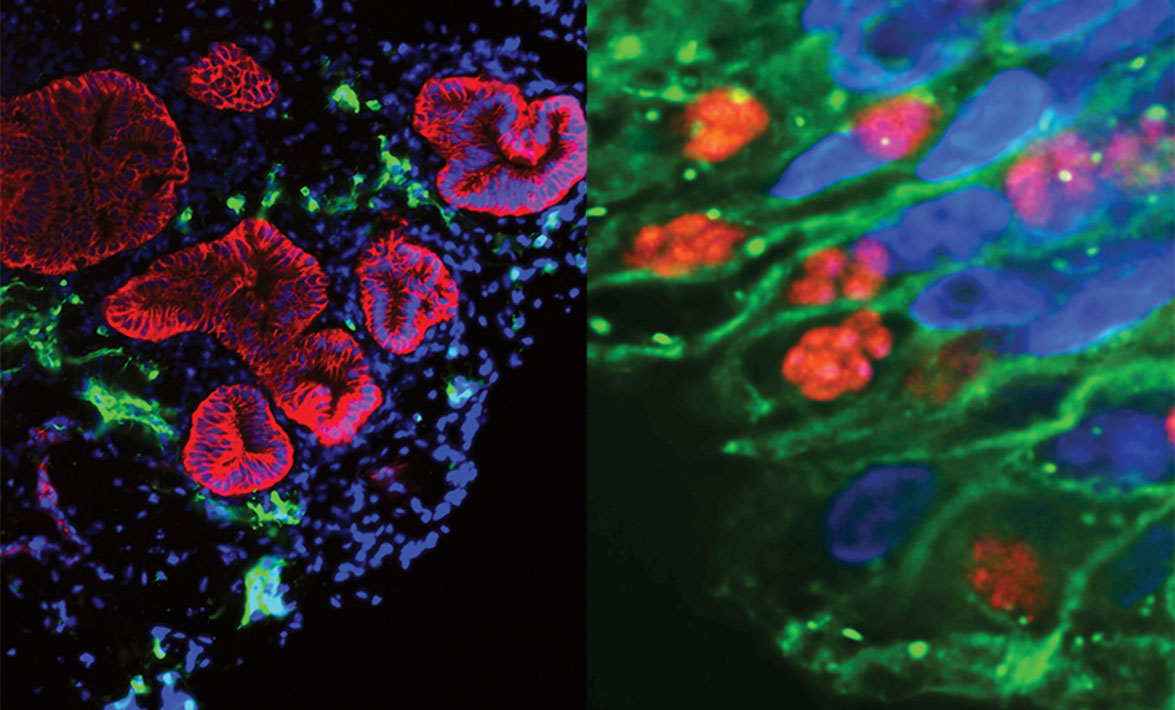
The whole process takes more than three months from beginning to end, yielding what looks at first glance like a slightly out-of-focus gray beachball when seen under a standard light microscope. That unremarkable-looking sphere, however, hides a complex biology. The enteroid contains more than a single cell type inflated into three dimensions. Besides the enterocytes found in a 2D cell culture, the enteroid contains neurons, glial cells (which provide structural and metabolic support for neurons), mucus-producing cells and smooth muscle cells—just as in a living small intestine. Fluorescent stains reveal a rainbow of cell types with the serpentine curves of the small intestine, complete with tiny fingers of villi protruding into the middle of the pseudo-intestine.
But when the researchers first saw what Toxoplasma did, they worried. Although the parasite infected the enteroid, it didn’t replicate. For some reason, the parasite wasn’t happy. Countless trips between the Lee and Coppens laboratories showed that adding some extra nutrients specifically for Toxoplasma helped the parasite begin to divide. So did giving the enteroid 24 hours to adjust to tiny variations in temperature and humidity in the Coppens Lab before it was infected.
One month after Coppens and Lee met in the Wolfe Street café, Julia Romano, PhD, a research associate in the Coppens Lab, infected the enteroid produced by Hojae with a lab stock of Toxoplasma that carried a fluorescent tag allowing her to easily find it under the microscope. But it would take 24 hours for her to know whether Hojae’s months of hard work would pay off. Romano impatiently watched the seconds tick by until she could place the enteroid under the microscope. The glow of green told her it had worked. She immediately emailed Hojae to tell him the good news.
Organoids of heart, lung and brain, as well as of the small intestine, have revolutionized the study of many human diseases, not just toxoplasmosis. Lee says that one of their biggest promises has been in the field of drug discovery, since these three-dimensional miniature organ models more closely match the complexity of human biology. “We’re not just one cell type,” Lee says. “Everything is all connected.”
In the 10 months since their first meeting in the café, Lee and Coppens have gathered preliminary data on how Toxoplasma behaves in an enteroid, and they're projected to submit a major NIH grant for more funding to use the system for drug development. Their collaboration may likewise answer some basic scientific questions about Toxoplasma and the parasite Cryptosporidium, both of which infect the body as dormant cysts and then transform into rapidly dividing forms in the cells of the small intestine. What triggers this transformation remains a complete mystery, Coppens says.
The reverse process is also unknown: Toxoplasma turns back into mostly inactive cysts in brain and muscle cells and can subsequently transform yet again into quickly dividing tachyzoites. No one knows what signals prompt these swaps, although Coppens and other scientists believe that host factors play a major role—one reason Lee’s enteroids are so important. If scientists can find a way to keep Toxoplasma cysts from becoming active, and thus remaining tucked away and harmless within the body, it would be a major advance for people with weakened immune systems.
Lee’s hopes for his enteroids extend beyond building models of the human small intestine. He would also like to model the feline small intestine so that scientists like Coppens' can study the sex life of Toxoplasma. Although researchers know that cat guts are where the magic happens for these single-celled parasites, the molecular details remain unclear. Thus far, however, Lee has not had the time and funding to obtain intestinal samples from Fluffy and convert them to enteroids.
Coppens says that while her work can seem far removed from the activities of other public health researchers, “public health includes basic science, too.” Studying the precise features of the vacuole that harbors Toxoplasma in the human host isn’t academic minutiae. These details may help researchers like her figure out what makes the parasite tick. This, in turn, gives scientists a chance to spike its plans with new, better treatments.
Lee is optimistic about those prospects. “Organoids will bring in a new era,” he says—an era already dawning in two Hopkins labs on either side of Wolfe Street.
